A global group of researchers is calling for better integration of viral genetics, bioinformatics, and public health to enable better pandemic response now and better pandemic preparedness in the future. In a comment piece in the journal Nature, an international collaboration of specialists in viral and genetic analysis, led by Swiss scientists Dr. Emma Hodcroft at the University of Bern and Prof. Christophe Dessimoz at University of Lausanne, both at the SIB Swiss Institute of Bioinformatics, alongside Dr. Nick Goldman at EMBL-EBI in the UK, lay out the ‘bioinformatics bottlenecks’ that are hindering response to the SARS-CoV-2 pandemic, and propose ways to ‘clear the road’ for better tools and approaches. Here are the key take home messages and perspective from the Swiss angle.

“What scientists have achieved in a year since the discovery of a brand-new virus is truly remarkable,” says Emma Hodcroft from the Institute of Social and Preventive Medicine (ISPM) of the University of Bern, first author on the piece, “but the tools scientists are using to study how SARS-CoV-2 is transmitting and changing were never designed for the unique pressures – or volumes of data – of this pandemic.”
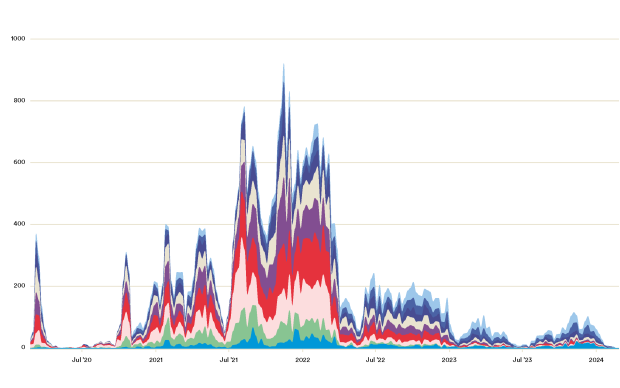
SARS-CoV-2 is now one of the most sequenced pathogens of all time, with over 600,000 full-genome sequences having been generated since the pandemic began, and over 5,000 new sequences coming in from around the world every day. However, the analysis and visualization tools used today (including Nextstrain, co-developed by Prof. Richard Neher's group at the SIB and the University of Basel) were never designed to handle the volume and speed of sequences being generated today, or the scale of the involvement with public health response. “Across the world, viral genomic surveillance rests on the initiative of academic researchers to find essential answers. Public health decision making would benefit from a more sustainable collaboration framework,” says Christophe Dessimoz at SIB and University of Lausanne.
What an improved sequencing would enable
The genetic sequences from SARS-CoV-2 hold valuable information to implementing effective pandemic policies and staying ahead of the virus. Comparing how many mutations different samples share, for example, allows scientists to track the transmission of the virus – helping to identify super-spreading events and international spread. But at the moment it can be hard to combine this genetic information with other key variables - like who attended an event, and when symptoms appeared – which could help make these methods even more informative.
The ‘R-number’ has gone from a scientific concept to a household word in the last year – it measures the average number of people an infected person will transmit to. Here, sequences can help too, by helping to pick apart imported cases from local transmission. This allows for a more accurate estimate of Re, but needs high levels of sequencing and complex analyses, which are currently not widely implemented.
Finally, sequencing is the only way to identify and track the many mutations that arise in SARS-CoV-2. While mutations are a normal part of virus life, scientists need to know which are harmless variations and which could change the virus’ transmissibility or clinical outcome. Combining sequences, lab work, and computational predictions could allow for a better understanding of mutational impacts, but there’s little framework to help these different specialities work together. “The viral data - sequences and associated metadata- must be determined, gathered and harmonized thanks to stable infrastructures compatible with the principles of open data to facilitate peer-review by the community and their reuse”, says Christophe Dessimoz at SIB and University of Lausanne, last author of the article.
Benefits for Switzerland
“In Switzerland, the population could benefit from more systematic and representative sequencing, for example through better contact tracing, targeted isolation and quarantine of smaller regions, and guiding the closing and opening of schools based on the presence of certain variants”, explains Emma Hodcroft.
Harmonisation of health data practices is also a critical topic. Switzerland is already putting a lots of efforts at the national level through the Swiss Personalized Health Network (SPHN). The researchers are convinced that Switzerland's potential in terms of expertise and infrastructure is just waiting to be tapped, to the benefit of public health. “The tools to enable research are there, and researchers have self-organized and taken the first step: to scale up and sustain these efforts to bring research and public health closer together, we rely on a sustainable public funding”, says Christophe Dessimoz.
Reference(s)
Emma B. Hodcroft, Nicola De Maio, Rob Lanfear, Duncan R. MacCannell, Bui Quang Minh, Heiko A. Schmidt, Alexandros Stamatakis, Nick Goldman & Christophe Dessimoz: : Want to track pandemic variants faster? Fix the bioinformatics bottleneck. Nature Comment, 1 March 2021.