The Swiss Personalized Health Network has launched a new program to integrate cantonal hospitals into its network. The tools provided will enable the hospitals to securely transfer interoperable data, paving the way for future research projects. Find out more…
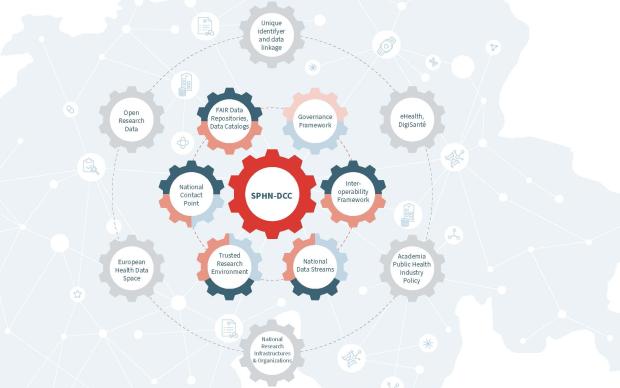
The Swiss Personalized Health Network is a national initiative launched by the State Secretariat for Education, Research and Innovation in 2017 under the responsibility of the Swiss Academy of Medical Sciences (SAMS). SPHN is managed by SAMS and SIB. Our institute brings its expertise to lead the Data coordination Center of the initiative as well as the BioMedIT network, the secure IT network for the responsible processing of health-related data. The aim of the initiative is to contribute to the development, implementation and validation of coordinated data infrastructures in order to make health-relevant data interoperable and shareable for research in Switzerland.
The Swiss Personalized Health Network (SPHN) has announced the launch of a new program designed to integrate cantonal hospitals into the SPHN and BioMedIT network. This initiative aims to extend and standardize data delivery across Swiss healthcare institutions, to enable these hospitals to participate in multi-site personalized medicine research in the future. The cantonal hospitals participating in this program include Cantonal Hospital Lucerne (LUKS), Cantonal Hospital Aarau (KSA), Ente Ospedaliero Cantonale (EOC), Cantonal Hospital Baden (KSB), Cantonal Hospital St.Gallen (KSSG) and the Swiss Group for Clinical Cancer Research (SAKK).
The Swiss Personalized Health Network is a national initiative launched by the State Secretariat for Education, Research and Innovation in 2017 under the responsibility of the Swiss Academy of Medical Sciences (SAMS). SPHN is managed by SAMS and SIB. Our institute brings its expertise to lead the Data coordination Center of the initiative as well as the BioMedIT network, the secure IT network for the responsible processing of health-related data. The aim of the initiative is to contribute to the development, implementation and validation of coordinated data infrastructures in order to make health-relevant data interoperable and shareable for research in Switzerland.
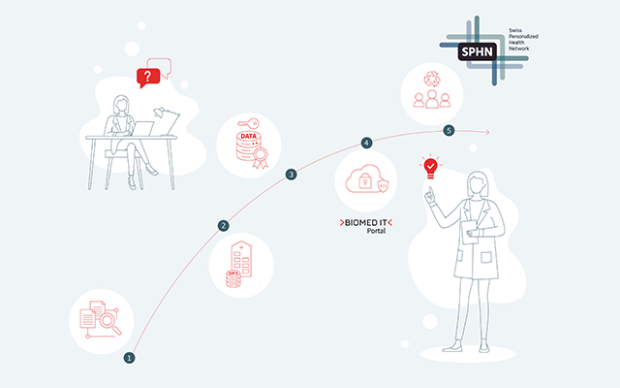
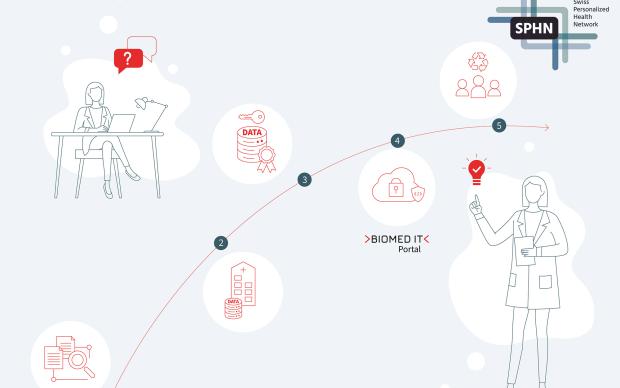
As part of the onboarding process, participating hospitals will map their internal data to international standards such as SNOMED CT (Systematized Nomenclature of Medicine - Clinical Terms) and LOINC (Logical Observation Identifiers Names and Codes). SNOMED CT and LOINC are globally recognized standards that ensure the consistent and accurate exchange of clinical data.
The minimal data set includes demographics, diagnoses, procedures, medications and lab tests, as well as key scores and vital signs. Using the SPHN Connector, the data is transformed into interoperable datagraph that can be understood both by users and computers. This is done by representing the data in a knowledge graph according to the SPHN Semantic Interoperability Framework. It ensures that the data follows the FAIR data principles, making the data findable, accessible, interoperable, and reusable, and facilitates seamless data integration and analysis.
By participating in the initiative, the cantonal hospitals are also setting up the IT-infrastructure needed for further collaboration. Along with the SPHN Connector, to transform the data, they also set up the Secure Encryption and Transfer Tool (sett) allowing for encryption and secure transfer of the data from the hospital to the trusted IT environment BioMedIT.
In a collaborative effort to enhance oncology patient data delivery, the program will also involve the Swiss Group for Clinical Cancer Research. It will be onboarded to provide a minimal dataset for oncology patients originating from many different hospitals across Switzerland in the SPHN format.
This program represents a significant step towards enlarging the SPHN network to standardized and efficient data sharing across Swiss healthcare institutions, promoting better healthcare outcomes through personalized medicine research.