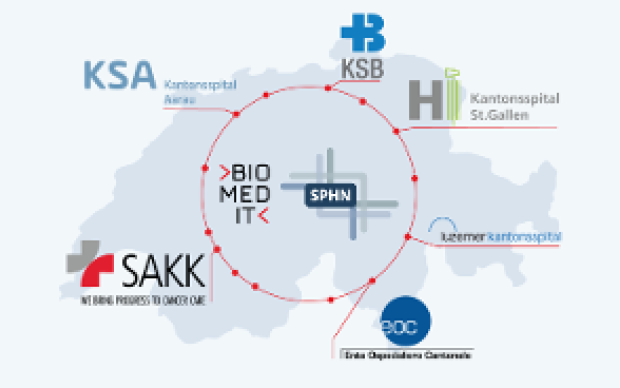
Health data from patients are typically generated and stored in a multitude of ways across Swiss hospitals. Making these data both accessible and useful to biomedical researchers is the goal of the Swiss Personalized Health Network initiative (SPHN). And to move from fragmented heterogeneous data to a harmonized pool of quality health information, a range of common standards and processes must be developed and adopted, from IT infrastructure to data ontologies. Nationwide expert groups – coordinated by the SIB Personalized Health Informatics Group (PHI) – have been created to this end: discover how they operate below.
In the role of the conductor: the PHI Group at SIB
The PHI Group is in charge of both the BioMedIT project and the SPHN Data Coordination Centre. One of its missions is thus to orchestrate the activities of the working groups and to ensure that they cover current and future needs pertaining to research infrastructure, through a continuous gap analysis. It also maintains strong ties at the international level with other national or broad data infrastructure initiatives (e.g. ELIXIR, GA4GH) to avoid reinventing the wheel if a solution to a particular challenge already exists and can be adapted to Swiss needs.
Interoperability: a prerequisite for data exchange
Obtaining access to consented patient data from a single hospital as part of a one-off research project is one thing. Enabling researchers to securely tap into the wealth of patient data from across Switzerland’s hospitals is another. “To ensure nationwide interoperability of biomedical data, we need to address a variety of technical and semantic challenges, ranging from the agreement on common data standards, to building a federated secure IT infrastructure,” explains SIB’s Sabine Österle, Scientific Project Manager at PHI. “Getting these changes to be adopted in the long run is a huge challenge. It is essential that they address the researchers’ needs – such as ease of use and access or low administrative burden. But it is equally essential that they fulfill IT security, ethical and legal constraints, and that their implementation remains practical on the hospitals’ side.”
Defining realistic guiding principles, in close dialogue with research
To tackle these challenges, the PHI Group at SIB sets up and coordinates expert working groups. Aiming to develop pragmatic and long-term ‘best practice toolboxes’ covering all key dimensions of interoperability in the SPHN, they collaborate closely with SPHN-funded projects – the ‘Driver projects’ – to ensure a close fit with the needs of researchers. Representing concrete cases of how patient data are used in research, Driver projects are also funded by the SPHN to ensure they act as beta-testers through very tight dialogue and exchange with the working groups.
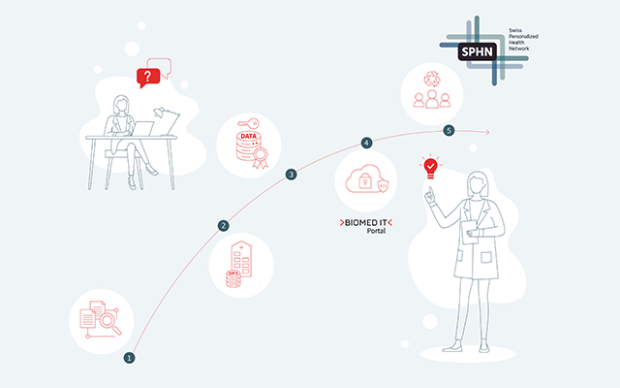
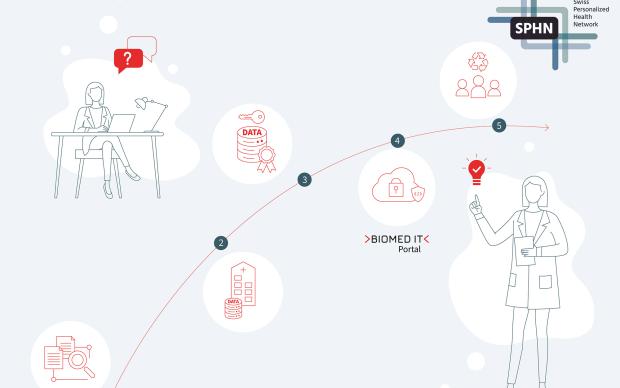
The process envisaged for clinical data sharing within the SPHN
First and foremost, within each hospital, all clinical data relevant for research are extracted from the primary systems and integrated into newly built, dedicated IT infrastructure solutions. A harmonized data catalogue, set up by HospIT for each university hospital, documents the data available for further research purposes. Once a researcher requests data according to the rules and procedures currently being defined by HospIT, the requested data are de-identified (pseudonymized/coded) in a consistent manner and assigned a Research ID. Next, the data are transformed into a common delivery format – also to be specified by HospIT – allowing for the transfer of diverse data types, from simple variables to large images. Using the encryption and transfer tool developed by the BioMedIT interoperability working group, the data are sent, in an encrypted form, from the hospital to BioMedIT nodes. A central Portal interface will give researchers access to both computational resources and project-specific data.
A multi-level approach, from clinical data semantics to IT infrastructure
How do these five national working groups operate? They each focus on a different aspect of interoperability and bring together the leading Swiss experts in each area.
More specifically, the question of data format and semantics is addressed by the Clinical Data Semantic Interoperability working group. “The challenge is to bridge two worlds: the world of health care and that of research. The former is producing large amounts of heterogeneous data: texts from medical reports, numbers from laboratory results, images, etc. To allow the research community to benefit from it, the objective is thus to move from transporting data, to transporting the interpretability of data, and thus to convey meaning. From the type of analyzer or kit used to the characteristics of the sample analyzed, all this information is required to properly interpret the results – not to mention the clinical context of the patients,” explains Christian Lovis, Head of the Medical Information Sciences Division of the Geneva University Hospitals (HUG) and chair of the group, which brings together medical technology and informatics specialists from Switzerland’s key health institutions. After identifying, together with the Driver projects, the clinical variables that will be essential for their work, the group proposes appropriate standards and definition in the form of SPHN datasets. These become available to the whole community. “Rather than creating and imposing new standards, our approach relies heavily on identifying the most suitable standards and format from existing (inter)national initiatives.”To develop the IT infrastructure of hospitals that will enable data sharing, the Hospital IT working group (HospIT) includes IT and knowledge management experts from the five Swiss university hospitals. “We focus on technical solutions and standards necessary for the harmonization and development of Swiss-wide IT infrastructure,” explains Cornelia Kruschel Weber, Chair of the working group and Head of Portfolio- and Project Management at the Research Data Service Centre of the University Hospital of Zurich. A key goal of the group is thus to enable the transfer of data, from the hospitals to the researchers via BioMedIT – the decentralized and secure IT infrastructure – in a harmonized way. This involves defining a set of rules and formats (see box).
This brings us to the third working group, BioMedIT interoperability, composed of IT and computer specialists who are looking into ways to share and analyze data within the SPHN network (see box). “Our aim is to provide a secure and protected IT environment fulfilling the stringent legal requirements that apply when using patient data,” says SIB’s Kevin Sayers, Chair of the working group. “This way, researchers can fully focus on their scientific research.” BioMedIT is currently being built on several computing nodes across Switzerland. “We are also developing the underlying infrastructure to enable workflow execution across the BioMedIT nodes, and are collaborating with the Swiss Data Science Centre (SDSC) to test the RENKU open data platform with biomedical analysis use cases,” explains Sayers.
The activities of two other working groups are also ramping up: the first is the BioMedIT Security working group, which aims to devise unified security measures for BioMedIT IT infrastructure. The second is the Bioinformatics and Data Analytics working group, which aims to provide use cases and recommendations on bioinformatics and data analytics.
“We are truly at a historical point in time,” concludes Katrin Crameri, Director of the PHI Group at SIB. “Thanks to tremendous collective work, Swiss health institutions and technology providers are working together towards the creation of – and agreement on – common standards and procedures, which will be of immense value to data-driven medicine.”