A comprehensive, long-term analysis of antibiotic resistance in bacteria shows that for many species, the proportion of resistant and non-resistant strains tends to reach an equilibrium over time – and that antibiotic use does not fully explain changes in drug resistance. The study by evolutionary and epidemiology experts, including members of two SIB Groups, provides new insights to help monitor and manage antimicrobial resistance.
Antibiotic resistance is not systematically increasing
Antibiotic resistance is a major public health concern, contributing to an estimated 5 million deaths per year. This health burden could increase further if resistant bacterial strains replace non-resistant strains within a species population. While a steady increase in the proportion of resistant strains could intuitively be expected through natural selection, the long-term evolutionary dynamics of antibiotic resistance are not well understood.
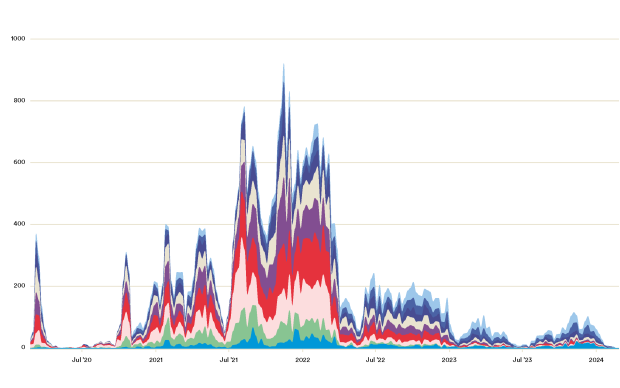
To gain insights into future resistance trajectories, a study co-led by SIB Group Leader Sonja Lehtinen from the University of Lausanne (UNIL) analysed a vast amount of data on drug resistance and antibiotic use in Europe using mathematical and statistical modelling. The dataset included resistance profiles of over 3.3 million clinical bacterial samples collected across 30 countries between 1998 and 2019, from eight bacteria species important to public health – including Streptococcus pneumoniae, Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae.
The researchers found that while the proportion of antibiotic-resistant strains within a particular species population initially rises in response to antibiotic use, it does not increase indefinitely. Instead, resistance rates in most species reached an equilibrium over the 20-year period.
Factors beyond antibiotic use may drive resistance
The study also showed that the rate of antibiotic resistance tends to increase faster, and reach a higher equilibrium level, in countries where antibiotic use is higher. However, a large fraction of the variability in resistance dynamics between countries could not be explained by antibiotic use, suggesting other factors may also play a role.
Insights for combating antimicrobial resistance
While the study has limitations – such as only covering Europe and not including all species where antibiotic resistance represents a significant public health problem – it nevertheless demonstrates the complexity of resistance evolution in the long term. This complements shorter-term approaches, such as yearly resistance reports generated by the European Center for Disease Prevention and Control.
The new insights show the potential impact of curbing antibiotic consumption in resistance management and could help to monitor and characterize the emergence of new resistances. The authors also highlight that combating the threat of antimicrobial resistance requires a better understanding of all factors involved in resistance evolution.
In addition to Sonja Lehtinen, the study also includes Martin Emons from the University of Zurich, a member of the SIB Group led by Mark Robinson.
Reference(s)
Emons M, Blanquart F, Lehtinen S (2025) The evolution of antibiotic resistance in Europe, 1998–2019. PLoS Pathog 21(4): e1012945.
Image: 3D computer-generated image of Streptococcus pneumoniae bacteria based on scanning electron microscopic imagery. Unsplash.